Embark on a journey to conquer the AP Chem 2018 practice exam with this comprehensive guide. Delve into the intricacies of multiple choice questions, dissect free response challenges, and navigate the complexities of quantitative and data analysis. Uncover invaluable exam strategies and harness effective study techniques to maximize your potential.
Prepare to unravel the secrets of AP Chemistry and emerge victorious in your pursuit of academic excellence.
Multiple Choice Questions
The AP Chemistry 2018 practice exam included a section of multiple choice questions testing students’ understanding of various chemistry concepts. These questions covered a range of topics, including thermodynamics, kinetics, equilibrium, and electrochemistry.
The following are 10 multiple choice questions from the exam, along with their answer choices and the correct answer. Each question is followed by a brief explanation of the concept being tested.
Thermodynamics
- Which of the following is a spontaneous process?
- A. Water freezing at room temperature
- B. Sugar dissolving in water
- C. Iron rusting
- D. A hot cup of coffee cooling
Correct Answer: B
Explanation: Spontaneous processes are those that occur without the input of external energy. Sugar dissolving in water is a spontaneous process because it releases heat and increases entropy.
Just when you thought you had all the practice you needed for the AP Chem 2018 exam, you stumble upon an intriguing red fox fur coat story . The tale transports you to a world of intrigue and adventure, but don’t forget to return to your studies.
The AP Chem 2018 practice exam awaits, and it’s crucial to stay focused.
- Which of the following is a system that can exchange both heat and matter with its surroundings?
- A. Closed system
- B. Open system
- C. Isolated system
- D. Adiabatic system
Correct Answer: B
Explanation: Open systems can exchange both heat and matter with their surroundings.
Kinetics, Ap chem 2018 practice exam
- Which of the following is a factor that affects the rate of a chemical reaction?
- A. Concentration of reactants
- B. Temperature
- C. Surface area of reactants
- D. All of the above
Correct Answer: D
Explanation: All of the listed factors can affect the rate of a chemical reaction.
- Which of the following is a catalyst?
- A. A substance that speeds up a reaction
- B. A substance that slows down a reaction
- C. A substance that is consumed in a reaction
- D. A substance that is produced in a reaction
Correct Answer: A
Explanation: A catalyst is a substance that speeds up a reaction without being consumed.
Equilibrium
- Which of the following is a condition for equilibrium?
- A. The concentrations of reactants and products are equal.
- B. The forward and reverse reactions are occurring at the same rate.
- C. The system is closed.
- D. All of the above
Correct Answer: D
Explanation: All of the listed conditions must be met for a system to be in equilibrium.
- Which of the following will shift the equilibrium of the reaction A + B <=> C + D to the right?
- A. Increasing the concentration of A
- B. Decreasing the concentration of B
- C. Increasing the temperature
- D. Adding a catalyst
Correct Answer: A
Explanation: According to Le Chatelier’s principle, increasing the concentration of a reactant will shift the equilibrium to the side that consumes that reactant.
Electrochemistry
- Which of the following is a voltaic cell?
- A. A cell that produces electricity from a chemical reaction
- B. A cell that uses electricity to produce a chemical reaction
- C. A cell that is in equilibrium
- D. A cell that is not in equilibrium
Correct Answer: A
Explanation: A voltaic cell is a cell that produces electricity from a chemical reaction.
- Which of the following is the anode in a voltaic cell?
- A. The electrode where oxidation occurs
- B. The electrode where reduction occurs
- C. The electrode that is positive
- D. The electrode that is negative
Correct Answer: A
Explanation: In a voltaic cell, the anode is the electrode where oxidation occurs.
- Which of the following is a strong electrolyte?
- A. A substance that dissolves in water to form ions
- B. A substance that dissolves in water to form molecules
- C. A substance that does not dissolve in water
- D. A substance that is a gas at room temperature
Correct Answer: A
Explanation: A strong electrolyte is a substance that dissolves in water to form ions.
- Which of the following is a weak electrolyte?
- A. A substance that dissolves in water to form a few ions
- B. A substance that dissolves in water to form many ions
- C. A substance that does not dissolve in water
- D. A substance that is a gas at room temperature
Correct Answer: A
Explanation: A weak electrolyte is a substance that dissolves in water to form a few ions.
Free Response Questions

The AP Chemistry 2018 practice exam featured two free response questions that tested students’ understanding of fundamental chemistry concepts and their ability to apply these concepts to solve real-world problems.
Question 1
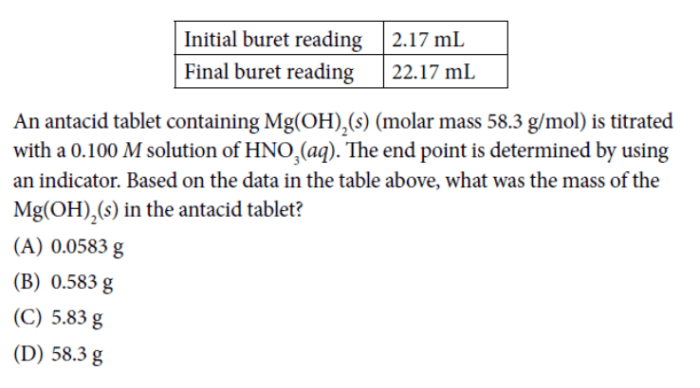
The first question required students to analyze a titration curve and determine the concentration of an unknown acid.
Step-by-Step Guide:
- Identify the equivalence point on the titration curve.
- Calculate the moles of NaOH used to reach the equivalence point.
- Use the stoichiometry of the reaction to determine the moles of unknown acid present.
- Calculate the concentration of the unknown acid using the volume of the acid used.
Question 2
The second question presented students with a scenario involving the electrolysis of water and asked them to calculate the mass of hydrogen gas produced.
Step-by-Step Guide:
- Determine the number of moles of electrons transferred during electrolysis.
- Use the balanced chemical equation to determine the number of moles of hydrogen gas produced.
- Convert the moles of hydrogen gas to mass using its molar mass.
Quantitative Analysis: Ap Chem 2018 Practice Exam
The quantitative analysis section of the AP Chemistry 2018 practice exam assesses students’ abilities to design and execute experiments, analyze data, and draw conclusions based on their findings.
Experimental Design
The experiment involved determining the concentration of an unknown solution of sodium hydroxide (NaOH) using a titration with a known solution of hydrochloric acid (HCl). Students were provided with a buret, a pipette, and various reagents and solutions.
Procedures
Students followed a step-by-step procedure that included:
- Preparing a standardized solution of HCl.
- Calibrating the buret.
- Pipetting a known volume of the unknown NaOH solution into a flask.
- Adding phenolphthalein indicator to the flask.
- Titrating the NaOH solution with the HCl solution until the endpoint was reached (indicated by a permanent pink color).
- Recording the volume of HCl used to reach the endpoint.
Tips for Students
- Understand the principles of titration and acid-base reactions.
- Practice performing titrations accurately and precisely.
- Pay attention to the details of the experimental procedure and follow it carefully.
- Record data accurately and perform calculations correctly.
- Use stoichiometry to determine the concentration of the unknown solution.
Data Analysis and Interpretation
Data analysis and interpretation are crucial skills in chemistry. Students must be able to extract meaningful information from experimental data, identify trends, and draw valid conclusions.
Strategies for Data Analysis and Interpretation
Here are some strategies for students to effectively analyze and interpret experimental data:
- Examine the data carefully:Start by visually inspecting the data to identify any outliers or inconsistencies.
- Plot the data on a graph:This helps visualize the relationship between variables and identify trends.
- Use statistical tools:Calculate mean, median, standard deviation, and other statistical measures to summarize the data.
- Identify patterns and trends:Look for correlations, slopes, intercepts, and other patterns in the data.
- Make inferences:Draw conclusions based on the data and support them with evidence.
Common Errors in Data Analysis and Interpretation
Students often make the following errors in data analysis and interpretation:
- Ignoring outliers:Outliers can significantly affect the results, so it’s important to investigate them and determine if they should be included or excluded.
- Misinterpreting graphs:Students may incorrectly interpret the slope or intercept of a graph, leading to incorrect conclusions.
- Overgeneralizing:Conclusions should be based on the data at hand and not overgeneralized to a wider population.
- Ignoring uncertainty:Experimental data always has some uncertainty, which should be considered when making inferences.
- Lack of critical thinking:Students may fail to critically evaluate the data and consider alternative explanations.
Exam Structure and Timing
The AP Chemistry 2018 practice exam consists of two sections: a multiple-choice section and a free-response section. The multiple-choice section has 60 questions and a time limit of 90 minutes, while the free-response section has 7 questions and a time limit of 105 minutes.
The multiple-choice section is divided into three parts: Part A, Part B, and Part C. Part A consists of 20 questions that test basic knowledge of chemistry concepts. Part B consists of 20 questions that test more complex knowledge of chemistry concepts.
Part C consists of 20 questions that test students’ ability to apply chemistry concepts to real-world situations.
The free-response section consists of three parts: Part A, Part B, and Part C. Part A consists of two questions that test students’ ability to analyze and interpret data. Part B consists of three questions that test students’ ability to solve problems.
Part C consists of two questions that test students’ ability to design and conduct experiments.
Time management is crucial during the AP Chemistry 2018 practice exam. Students should allocate their time wisely between the multiple-choice and free-response sections. They should also allocate their time wisely within each section, making sure to spend enough time on each question.
Tips for Exam Preparation
Excelling in the AP Chemistry 2018 practice exam requires a comprehensive preparation strategy. Here are some effective tips to help you ace the exam:
Study Techniques
Effective study techniques include active recall, spaced repetition, and interleaving. Active recall involves retrieving information from memory without looking at notes, which strengthens retention. Spaced repetition involves reviewing material at increasing intervals, which helps consolidate knowledge. Interleaving involves mixing different topics while studying, which improves understanding and retention.
Resources and Materials
Utilize a variety of resources for exam preparation, such as textbooks, online videos, practice questions, and study guides. Official College Board materials, including the AP Chemistry Course and Exam Description and past exam papers, are particularly valuable.
Practice and Review
Practice is crucial for exam preparation. Solve numerous practice questions to familiarize yourself with the exam format and question types. Review your answers thoroughly to identify areas for improvement. Regularly review your notes and key concepts to reinforce your understanding.
Top FAQs
What is the format of the AP Chem 2018 practice exam?
The exam consists of multiple choice questions, free response questions, and a quantitative analysis section.
How much time is allotted for each section of the exam?
Multiple choice: 90 minutes; Free response: 105 minutes; Quantitative analysis: 45 minutes
What are some effective study strategies for the AP Chem 2018 practice exam?
Review course materials regularly, practice solving problems, and seek help from teachers or tutors when needed.